I-Sodium Hypochlorite generator
I-Sodium Hypochlorite generator,
,
Incazelo
I-membrane electrolysis sodium hylochlorite generator iwumshini ofanele wokuphuza imali yokudlalisa imali, ukwelashwa kwamanzi ama-wasteway kanye ne-exstrow, kanye ne-Yantai University, i-yantai University, i-Yantai University, i-yantai University, i-Yantai University kanye namanye ama-maxion. I-membrane sodium hypochlorite generator yakhelwe futhi yakhiqizwa yi-Yantai Jietong Water Treatment Technology Co, Ltd ingakhiqiza isixazululo esingu-5-12% sokuhlushwa i-sodium hypocrorite solution nge-loop evaliwe yokukhiqiza ukusebenza okuzenzakalelayo.

Umgomo Wokusebenza
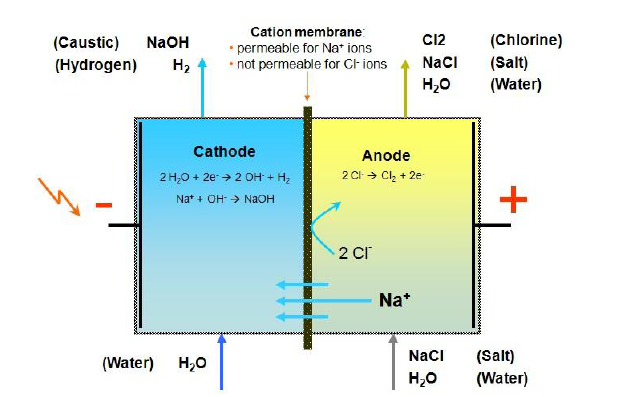
Isisekelo esiyisisekelo sokusabela kwe-electrone ye-membrane electrone cell ukuguqula amandla kagesi abe amandla kagesi kanye ne-electroly brine ukukhiqiza i-naoh, cl2 ne-H2 njengoba kukhonjisiwe kulesi sithombe esingenhla. Ku-anode Chamber of the cell (ngakwesokunene kwesithombe), i-brine inezinto ze-NA + futhi i-Cl- esitokisini, lapho kufudukela eChiniode Chamber (uhlangothi lwesobunxele lwesithombe) ngolwelwesi olukhethiwe lwe-ionic ngaphansi kwesenzo sokushaja. Ama-cleati aphansi akhiqiza igesi ye-chlorine ngaphansi kwe-anodic eyezempilo. I-H2O Ionization egumbini laseKathode liba yi-H + no-Oh-, lapho kuvinjelwa ulwelwesi lwe-cathode egumbini laseKathode nase-NA + kusuka ku-anode Chamber kuhlanganiswe nokwenza umkhiqizo we-Naoh, futhi kwakha i-hydrogen ngaphansi kwe-cathodic eyefikhiri.

Isicelo
● Imboni ye-chlorine-alkali
● Ukubulala amagciwane ngesitshalo samanzi
● Ukuqhuma kwezingubo zokwenza izingubo
● Dingiting ku-chlorine ephansi esebenzayo ye-chlorine yekhaya, ihhotela, esibhedlela.
Amapharamitha Prameters
| Isifanekiso
| Itlorine (kg / h) | UNaclo (kg / h) | Ukusetshenziswa usawoti usawoti (kg / h) | Amandla we-DC Ukusetshenziswa (Ww.H.H) | Indawo yokuhlala (㎡) | Ubunzima (Amathani) |
| JTWL-C1000 | 1 | 10 | 1.8 | 2.3 | 5 | 0.8 |
| JTWL-C5000 | 5 | 50 | 9 | 11.5 | 100 | 5 |
| JTWL-C10000 | 10 | 100 | 18 | 23 | -Mashumi | 8 |
| JTWL-C15000 | 15 | I-150 | 27 | 34.5 | -Mashumi | 10 |
| Jtwl-c20000 | 20 | -Mashumi | 36 | 46 | 350 | 12 |
| JTWL-C30000 | 30 | 300 | 54 | 69 | 500 | 15 |
Icala lephrojekthi
I-Sodium Hypochlorite generator
8Tons / Day 10-12%

I-Sodium Hypochlorite generator
I-200KG / Usuku 10-12%
 Ukuqalisa
Ukuqalisa
I-membrane electrolysis sodium hylochlorite generator iwumshini ofanele wokuphuza imali yokudlalisa imali, ukwelashwa kwamanzi ama-wasteway kanye ne-exstrow, kanye ne-Yantai University, i-yantai University, i-Yantai University, i-yantai University, i-Yantai University kanye namanye ama-maxion. Kuluhlobo lomshini wokukhiqiza izixazululo eziphakeme ze-sodium hypochlorite esizeni, wanelisa kakhulu isidingo semikhiqizo ephezulu ye-sodium hypochlorite, futhi ixazulule izinkinga zokuhamba nezinkinga zokugcina. I-membrane sodium hypochlorite generator ekhiqizwe yi-yantai jietong water Treatment Technology Co, Ltd. yinkampani kuphela yezobuchwepheshe eChina ezingakhiqiza imikhiqizo ephezulu ye-sodium hypocchorite ecaleni. UMembrane Electrolysis Brine Sodium Hypochlorite Generator angakhiqiza isixazululo esingu-5-12% esiphakeme se-sodium hypocchorite ngesisombululo esivaliwe se-dosing futhi sikhiqize ukusebenza okuzenzakalelayo okugcwele.
Isisekelo esiyisisekelo sokusabela kwe-electrone ye-membrane electrone cell ukuguqula amandla kagesi abe amandla kagesi kanye ne-electroly brine ukukhiqiza i-naoh, cl2 ne-H2 njengoba kukhonjisiwe kulesi sithombe esingenhla. Ku-anode Chamber of the cell (ngakwesokunene kwesithombe), i-brine inezinto ze-NA + futhi i-Cl- esitokisini, lapho kufudukela eChiniode Chamber (uhlangothi lwesobunxele lwesithombe) ngolwelwesi olukhethiwe lwe-ionic ngaphansi kwesenzo sokushaja. Ama-cleati aphansi akhiqiza igesi ye-chlorine ngaphansi kwe-anodic eyezempilo. I-H2O Ionization egumbini laseKathode liba yi-H + no-Oh-, lapho kuvinjelwa ulwelwesi lwe-cathode egumbini laseKathode nase-NA + kusuka ku-anode Chamber kuhlanganiswe nokwenza umkhiqizo we-Naoh, futhi kwakha i-hydrogen ngaphansi kwe-cathodic eyefikhiri.









